Funding to support U.S. commercial launch and global expansion
Mainstay Medical Holdings plc (“Mainstay” or the “Company”) today announced the closing of an equity financing in which it raised gross proceeds of US$108 million. Mainstay intends to use the funds to support the company’s commercial launch of ReActiv8® in the U.S., continued expansion in Europe and Australia, additional post-market clinical studies and research, and general operations.
The financing was co-led by new investors Ally Bridge Group and Sofinnova Partners, through its Crossover Fund, and also included a large, global medical device company. Key existing investors who participated in the financing include Sofinnova Partners (Capital Fund), KCK Group and Fountain Healthcare Partners.
Jason Hannon, CEO of Mainstay, commented: “A financing of this magnitude, supported by such a quality global investor group, is testament to the confidence in the commercial opportunity for ReActiv8. We are now strongly capitalized to execute on our corporate objectives in 2021 and beyond, including the launch of ReActiv8 in the U.S. market and acceleration of our commercialization efforts in Europe and Australia.”
“This is an exciting time for Mainstay as they bring to market a restorative therapeutic option for patients suffering from disabling chronic low back pain,” said Charles Chon, Partner and Managing Director at Ally Bridge Group, who also joins the Mainstay Medical Board of Directors. “We commend the Company on all the progress it has achieved to-date and look forward to supporting it going forward.”
Cédric Moreau, Partner at Sofinnova Partners, who also joins the Company’s Board of Directors, commented: “We are thrilled to co-lead such a strong syndicate of investors in fuelling Mainstay’s commercial acceleration to make its first-in-class neurostimulation technology available in the U.S. and more extensively worldwide.”
An extraordinary general meeting of Mainstay shareholders was held on 9 February 2021 to approve the financing and related matters. At the EGM, all resolutions were duly passed. The results of the voting on each of the resolutions is available on the Company’s website.
About Mainstay
Mainstay is a medical device company focused on commercializing an innovative implantable restorative neurostimulation system, ReActiv8®, for people with disabling mechanical Chronic Low Back Pain (“CLBP”). The Company is headquartered in Dublin, Ireland and has subsidiaries operating in the United States, Australia, Germany and the Netherlands.
About ReActiv8
ReActiv8 is an active implantable medical device designed to treat adults with intractable chronic low back pain associated with dysfunction of the lumbar multifidus muscle, a key stabilizing muscle of the low back, as evidenced by imaging or physiological testing in adults who have failed therapy, including pain medications and physical therapy, and are not candidates for spine surgery. ReActiv8 provides bilateral electrical stimulation of the L2 medial branch of the dorsal ramus nerve as it crosses the transverse process at L3. This nerve supplies the multifidus muscle to elicit contraction of the muscle which can lead to restoration of control over time, allowing the back to recover from CLBP.
ReActiv8 has a CE Mark allowing for commercialization in the European Economic Area and has been focused on building clinical validation in Germany in select centers ahead of wider commercial availability. ReActiv8 has also been admitted to the Australian Register of Therapeutic Goods (ARTG), enabling commercialization throughout Australia, and has been approved for inclusion on the Protheses List of reimbursed products in Australia, effective as of 1 July 2020. The Prostheses List identifies implantable devices eligible for reimbursement from all private health insurance funds in Australia. In the U.S., ReActiv8 is FDA approved and the Company plans to commercially launch in early 2021.
About Chronic Low Back Pain
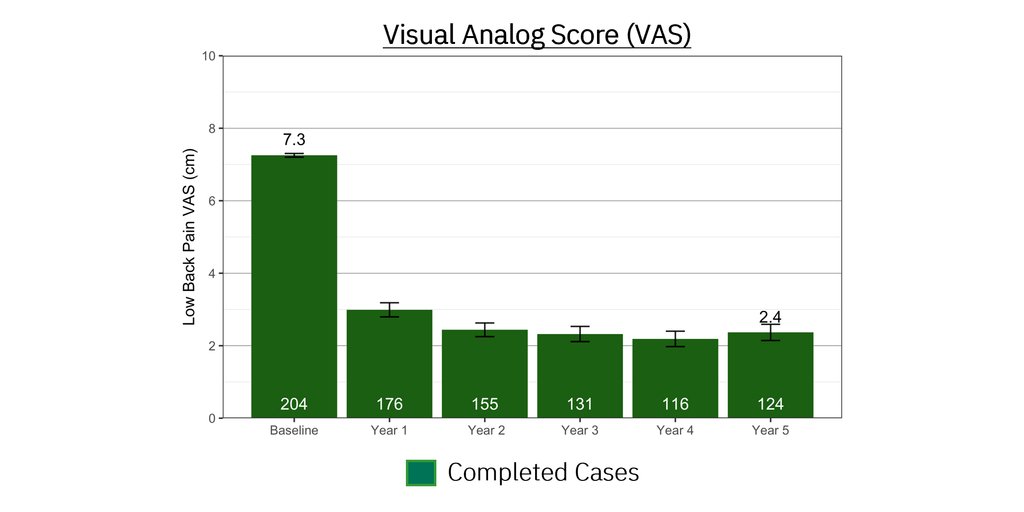
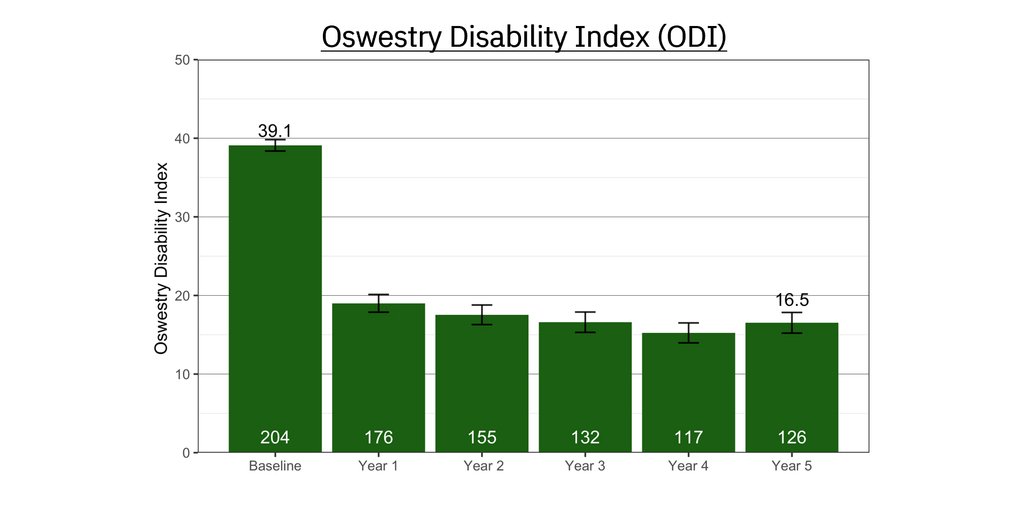
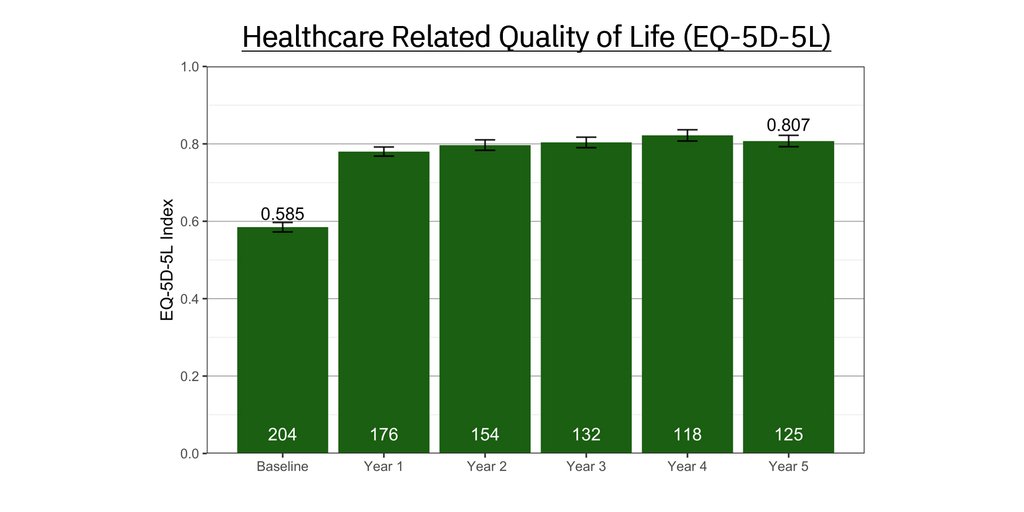
One of the root causes of CLBP is impaired control by the nervous system of the muscles that dynamically stabilize the spine. ReActiv8 is designed to electrically stimulate the nerves responsible for contracting these muscles to improve dynamic spine stability, allowing for improvement in CLBP and its disabling effects.
People with CLBP usually have a greatly reduced quality of life and score significantly higher on scales for pain, disability, depression, anxiety and sleep disorders. Their pain and disability can persist despite the best available medical treatments, and only a small percentage of cases result from an identified pathological condition or anatomical defect that may be correctable with spine surgery. Their ability to work or be productive is seriously affected by the condition and the resulting days lost from work, disability benefits and health resource utilization put a significant burden on individuals, families, communities, industry and governments.
Further information can be found at www.mainstay-medical.com
Forward looking statements
This announcement includes statements that are, or may be deemed to be, forward looking statements. These forward looking statements can be identified by the use of forward looking terminology, including the terms “anticipates”, “believes”, “estimates”, “expects”, “intends”, “may”, “plans”, “projects”, “should”, “will”, or “explore” or, in each case, their negative or other variations or comparable terminology, or by discussions of strategy, plans, objectives, goals, future events or intentions. These forward looking statements include all matters that are not historical facts. They appear throughout this announcement and include, but are not limited to, statements regarding the Company’s intentions, beliefs or current expectations concerning, among other things, the Company’s plans to commercialize ReActiv8 in the United States, the U.K., Australia and elsewhere; the commercial performance of ReActiv8; and the Company’s results of operations, financial position, prospects, financing strategies, expectations for product design and development, regulatory applications and approvals, reimbursement arrangements, costs of sales and market penetration and other commercial performance.
By their nature, forward looking statements involve risk and uncertainty because they relate to future events and circumstances. Forward looking statements are not guarantees of future performance, and actual results may differ materially from those described in, or suggested by, the forward looking statements contained in this announcement. In addition, even if future results and developments are consistent with the forward looking statements contained in this announcement, those results or developments may not be indicative of results or developments in subsequent periods. A number of factors could cause results and developments of the Company to differ materially from those expressed or implied by the forward looking statements, including, without limitation, the successful launch and commercialization of ReActiv8, general economic and business conditions, global medical device market conditions, industry trends, competition, the availability and cost of capital, changes in law or regulation, changes in taxation regimes, the time required to commence and complete clinical trials, the time and process required to obtain regulatory approvals, currency fluctuations, changes in its business strategy, and political and economic uncertainty. The forward-looking statements herein speak only at the date of this announcement.